The core principle behind why mercury lamps emit ultraviolet (UV) rays lies in the process where mercury atoms inside the lamp, after ionization and excitation, release energy at specific wavelengths.
Below is a detailed, step-by-step explanation of this process:
1. Core Physical Principle: Atomic Energy Levels and Light Emission
lEnergy Levels: Electrons in an atom cannot occupy arbitrary positions freely; instead, they can only exist in a series of discrete, specific energy states known as "energy levels."
lExcitation: When the mercury lamp is energized, free electrons inside the lamp tube are accelerated by the electric field, gaining high kinetic energy. These high-speed electrons collide with mercury atoms.
lTransition and Light Emission: During the collision, energy is transferred to the mercury atoms, causing their outer-shell electrons to "jump" from lower energy levels to higher ones—a process called "excitation." However, electrons in higher energy levels are highly unstable and will spontaneously jump back to lower energy levels very quickly (in approximately 10⁻⁸ seconds).
lPhoton Emission: When an electron jumps from a higher energy level to a lower one, the energy difference between the two levels is released in the form of a single photon. The energy (E) of the photon directly determines the wavelength (λ) of the light, following the formula E = hc/λ (where h is Planck’s constant and c is the speed of light). The higher the energy, the shorter the wavelength.
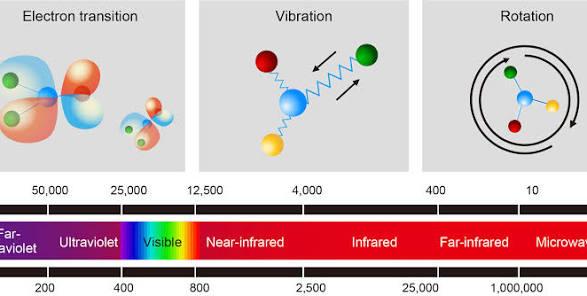
2. Unique Property of Mercury Atoms: Main Radiation in the UV Range
Mercury atoms have a unique energy level structure. When their electrons undergo transitions, the released energy corresponds exactly to specific wavelengths—among which the most prominent and intense spectral lines fall in the ultraviolet region.
The main characteristic spectral lines emitted by mercury atoms during discharge include:
l185nm (vacuum ultraviolet, VUV)
l254nm (short-wave ultraviolet, SWUV, with the strongest bactericidal effect)
l365nm (long-wave ultraviolet, LWUV, commonly known as UVA)
lSeveral visible light spectral lines, such as 435.8nm (blue), 546.1nm (green), and 579nm (yellow).
Essentially, the light emitted by mercury lamps inherently contains a large amount of ultraviolet rays—this is determined by the intrinsic physical properties of mercury atoms.
3. Different Types of Mercury Lamps and UV Ray Handling
Depending on the material of the lamp tube, these UV rays have different "fates," leading to the formation of various types of mercury lamps:
a) Low-Pressure Mercury Lamps (e.g., Germicidal Lamps)
lLamp Tube Material: Special glass that transmits UV rays (e.g., quartz glass) is used.
lWorking Principle: Under low pressure, the energy generated by the discharge of mercury vapor is mainly concentrated in two intense UV wavelengths: 253.7nm and 185nm. Ordinary glass absorbs these UV rays, but quartz glass allows them to transmit effectively.
lApplications: These UV rays are directly used for sterilization and disinfection (253.7nm can damage the DNA/RNA of microorganisms) or photochemical reactions.

b) High-Pressure/Ultra-High-Pressure Mercury Lamps (e.g., Mercury Lamps for Lighting)
lLamp Tube Material: Ordinary borosilicate glass is used.
lWorking Principle:
1. Under high pressure, the spectral lines of mercury broaden; additionally, due to the increased atomic density and enhanced intermolecular interactions, continuous background radiation is generated.
2. Ordinary glass strongly absorbs UV rays with wavelengths shorter than 300nm, especially the lethal short-wave UV rays.
3. Therefore, although UV rays are generated inside the tube, the glass envelope acts like a filter: it blocks most harmful short-wave UV rays and only allows long-wave UV rays (e.g., 365nm), visible light, and a small amount of infrared radiation to pass through.
lApplications: Primarily used for lighting, but with poor color rendering (dominated by blue-green light). The long-wave UV rays they emit can be used for fluorescence analysis, insect trapping, etc.
c) Fluorescent Lamps (Most Common Application)
lLamp Tube Structure: Their core is a low-pressure mercury lamp, but the inner wall of the tube is coated with a layer of phosphor.
lWorking Principle:
1. The discharge of mercury vapor mainly generates UV rays at 254 nm.
2. These UV rays irradiate the phosphor, which absorbs the UV energy and becomes excited itself.
3. When the phosphor returns from the excited state to the ground state, it releases the energy in the form of light with longer wavelengths and lower energy—i.e., visible light.
lApplications: By selecting different phosphor formulations, fluorescent lamps of various colors (e.g., "cool white light," "warm white light") can be produced. This realizes the efficient conversion of invisible UV rays into visible light, which is used for daily lighting.
Summary
lFundamental Cause: Mercury lamps emit UV rays because the energy released by electron transitions in excited mercury atoms corresponds exactly to the wavelengths of the UV spectrum—this is an inherent physical property of the mercury element.
lKey Factor: The pressure of mercury vapor inside the lamp tube determines the specific spectral line distribution of the UV rays.
lFinal Output: The material of the lamp tube (whether it transmits UV rays) and the presence of a phosphor coating determine whether these UV rays are utilized, blocked, or converted into visible light.
In simple terms, mercury lamps are inherently "UV generators." We use various technical methods to "control" these UV rays, enabling them to serve different purposes such as sterilization and lighting
Post time:2025-10-21 15:11:26

